Batteries and similar devices accept, store, and release electricity on demand. Batteries use chemistry, in the form of chemical potential, to store energy, just like many other everyday energy sources. For example, logs store energy in their chemical bonds until burning converts the energy to heat.
Gasoline is stored chemical potential energy until it is converted to mechanical energy in a car engine. Similarly, for batteries to work, electricity must be converted into a chemical potential form before it can be readily stored.
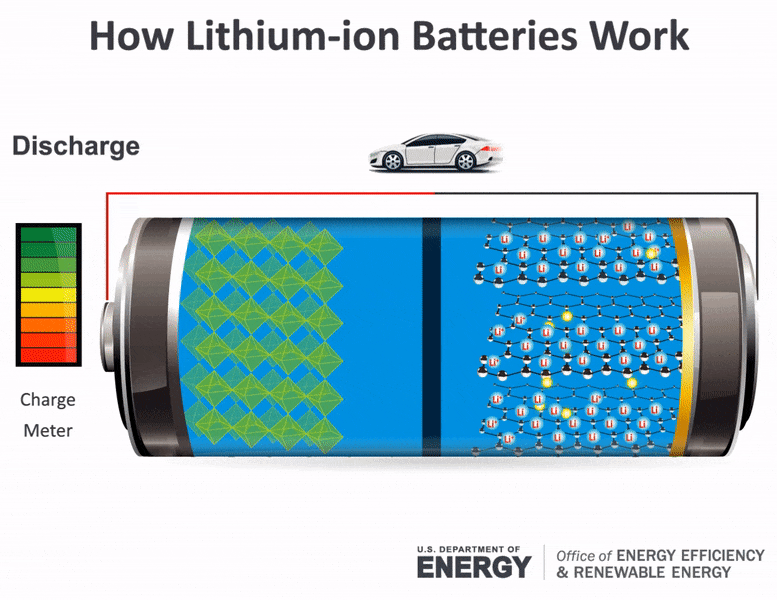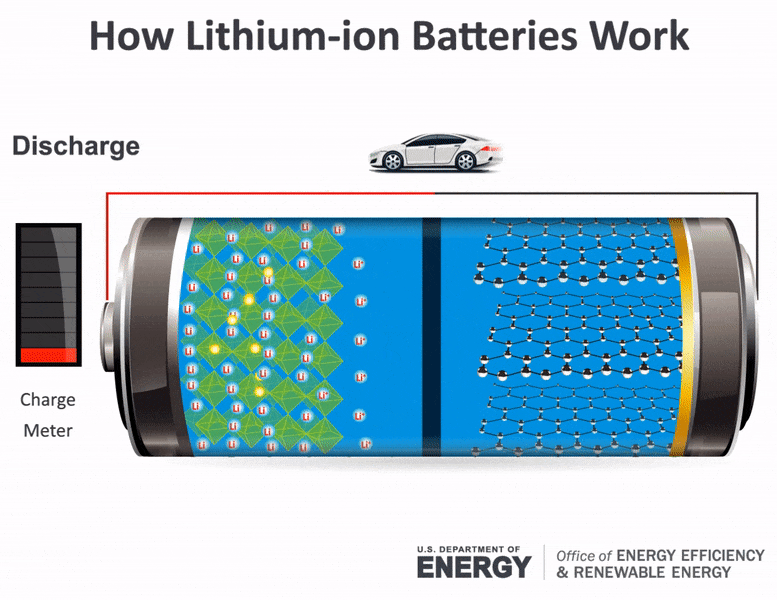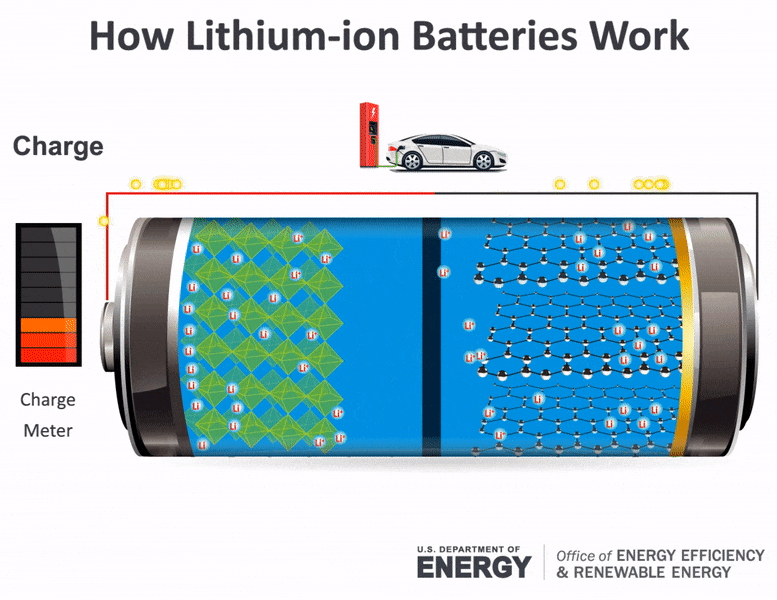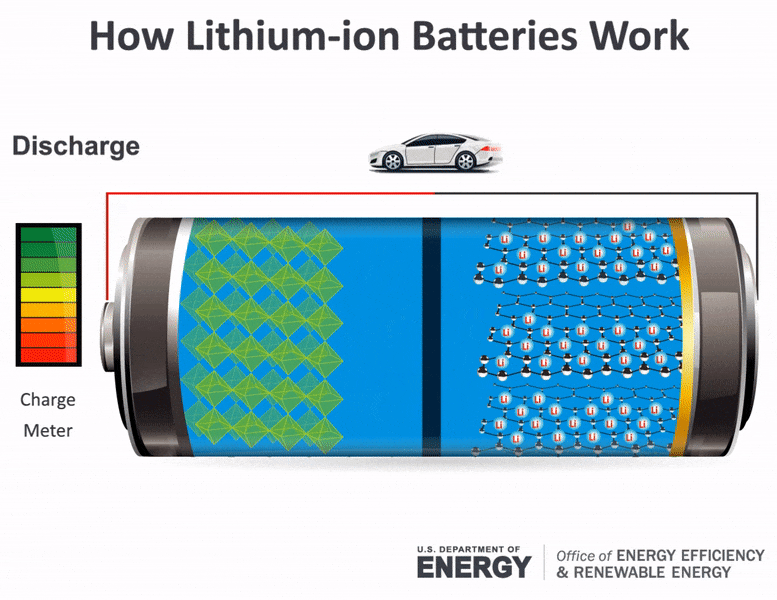
Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. To accept and release energy, a battery is coupled to an external circuit. Electrons move through the circuit, while simultaneously ions (atoms or molecules with an electric charge) move through the electrolyte.
In a rechargeable battery, electrons and ions can move either direction through the circuit and electrolyte. When the electrons move from the cathode to the anode, they increase the chemical potential energy, thus charging the battery; when they move the other direction, they convert this chemical potential energy to electricity in the circuit and discharge the battery. During charging or discharging, the oppositely charged ions move inside the battery through the electrolyte to balance the charge of the electrons moving through the external circuit and produce a sustainable, rechargeable system. Once charged, the battery can be disconnected from the circuit to store the chemical potential energy for later use as electricity.
Batteries were invented in 1800, but their chemical processes are complex. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. Scientists study processes in rechargeable batteries because they do not completely reverse as the battery is charged and discharged. Over time, the lack of a complete reversal can change the chemistry and structure of battery materials, which can reduce battery performance and safety.
Original article : Link






